Leafminers (Liriomyza spp.)
Credit: Biovision-Infonet

(c) A.M. Varela, icipe
Leafmining flies make tiny punctures on the side of tomato leaves when feeding and laying eggs. These punctures may serve as entry point for disease causing organisms such as bacteria and fungi.
The most destructive stages are the immature stages (maggots). Maggots measure up to 3 mm in length. The maggots feed on leaf tissue inside the leaf leaving a wandering track in the form of irregular mines. Heavy mining of leaves may reduce photosynthesis affecting development of flowers and fruits. Heavy attack may cause leaf drop. This is particularly serious for tomato seedlings which may die due to defoliation. Defoliation of tomato plants may also expose fruits to sunburn and affect the market value. Leafminers attack a wide range of cultivated vegetables.

(c) A. M. Varela, icipe
| What to do: Conserve natural enemies. Parasitic wasps normally control leafminers. However, the widespread indiscriminate use of persistent broad-spectrum insecticides, to control this and other pests, disrupt the natural control, leading to leafminer outbreaks. Rotate with non-host crops and plan the arrangement of fields so that old infested fields do not provide a reservoir of infestation for subsequent crops. Destroy leafminer pupae in the soil. This can be done by ploughing and tilling, by solarisation, and, on heavy soils, by flood irrigation. Monitor the crop by checking foliage for the presence of stipples caused by the adults while feeding and laying eggs, and for mines and larvae. Trap adult flies with yellow sticky or water traps. If necessary spray with neem-based pesticides. For more information on Neem-based pesticides click here. |
Leafminer (Liriomyza spp.) symptoms on Onions
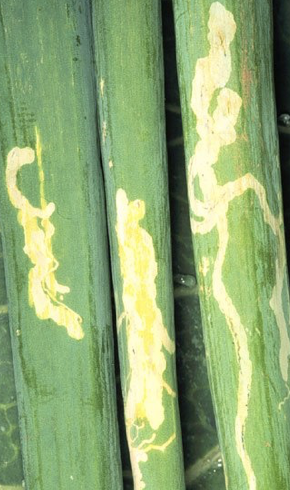
Leafminers may cause damage to green onions. Damage is largely cosmetic, and mining on leaves may cause rejection of marketed onions, but generally does not affect plant growth. Damage in dry onions and garlic is of little concern unless populations become high to prematurely kill foliage.What to do:
- Leafminers are usually controlled by natural enemies, especially parasitic wasps. They can become a problem in areas with a high use of pesticides that kill natural enemies. Leafminers have the ability to develop resistance to pesticides in a short time.












